
 |
|
d is used to mean ' a little bit of '
Di - ' 2 '
Pole - poles (positive and negative)
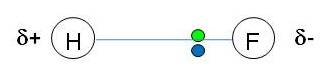
Polar molecules:
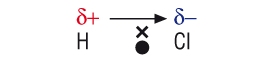
How is electronegativity measured?
Basically as you go towards the top right hand side of the Periodic table, the elements become more electronegative:
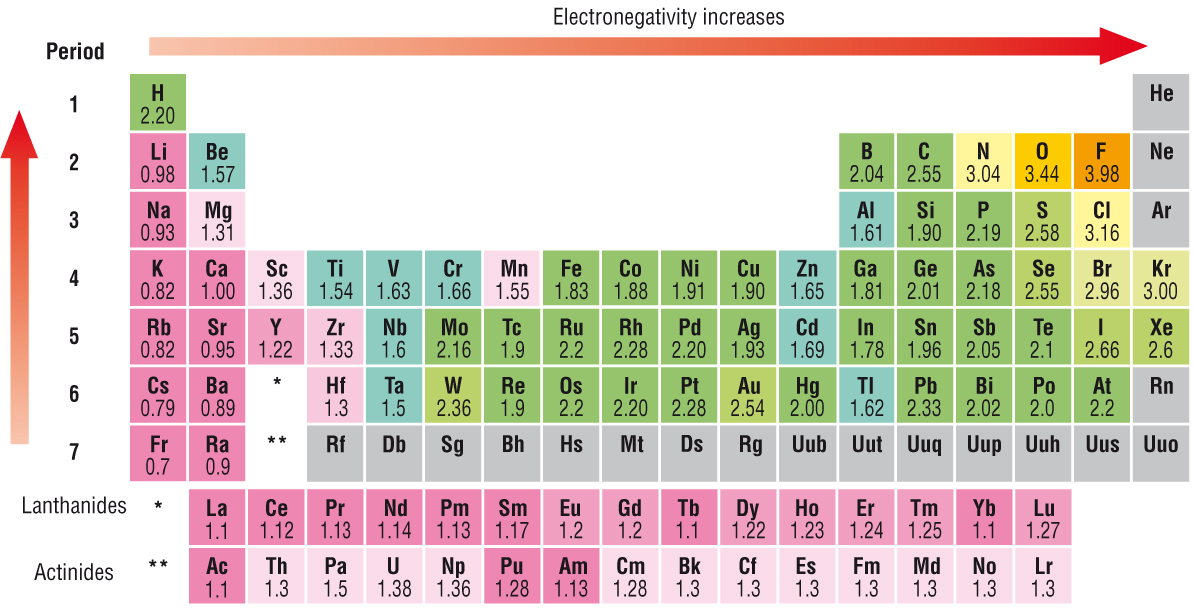
The electronegativity of an atom represents the power of an atom in a molecule to attract electrons to itself.
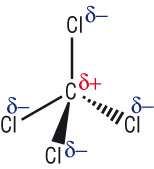
Electronegativity and bonding type
![]()
1) Covalent
2) Polar covalent
3) Ionic

Covalent to ionic:
Questions 1-2 P61
Strengths of bonds and forces:
| NaCl(s) |
|
Na+(g) | + | Cl-(g) |
| H2O(l) |
|
H2O(g) |
Since the molecules do not break up there must be some forces of attraction between the molecules, which are broken.
These are called intermolecular forces.
Since molecular substances can exist as solids, liquids and gases by varying the temperature and pressure, intermolecular forces must always exist although they may be very weak.
There are 3 types of intermolecular forces of attraction:
1) Van der Waals' forces
2) Permanent dipole - dipole forces
3) Hydrogen bonding
Permanent dipole - dipole interactions:
When we have 2 atoms in a covalent bond with different electronegativities, the bond is polarised.
If the molecule has a d+ end and a d- end, the molecule is said to have a permanent dipole:

The d+ end of one molecule will be attracted to the d- end of a neighboring molecule.
This attraction is called a permanent dipole - dipole force of attraction:

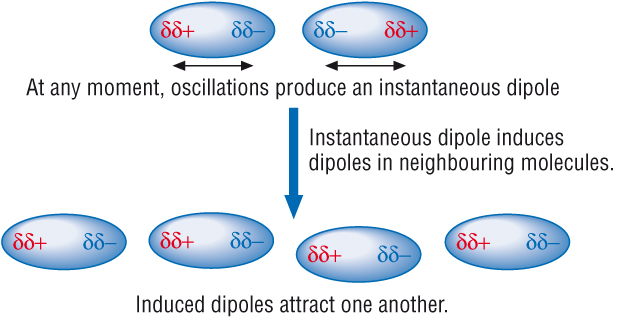
Van der Waals' forces (induced dipole - dipole interactions)
Helium does not form ionic or covalent bonds but it is possible to condense it to a liquid then to a solid.
Energy is released when a change of state occurs. 0.105KjMol-1
This very weak force of attraction is known as Van der waals forces.
It is due to the continually changing electric charge interactions between atoms, called induced dipole - dipole forces of attraction.
What causes Van der Waals' forces:
These are present in all molecules but are the only forces of attraction present in non - polar molecules
The electrons in shells are continually moving.
In the turmoil we get an uneven distribution of electrons / charge.
At any moment or snap shot in time there would be an instantaneous dipole across the whole atom / molecule.
The negative end of the dipole induces a dipole of opposite charge in neighbouring atoms
A force of attraction results.
These induced dipole – dipole interactions produces a cohesive force.
 |
|
Hydrocarbons Diatomic elements
The greater the number of electrons (and protons) in an atom / molecule, the greater these fluctuations are and the greater the fluctuations
This will give greater VDW attraction.
If you consider the alkanes – The boiling point increases as the Mr increases.
This is because of the increased number of electrons, which increase the VDW attraction.
Questions 1-2 P63
Water is peculiar
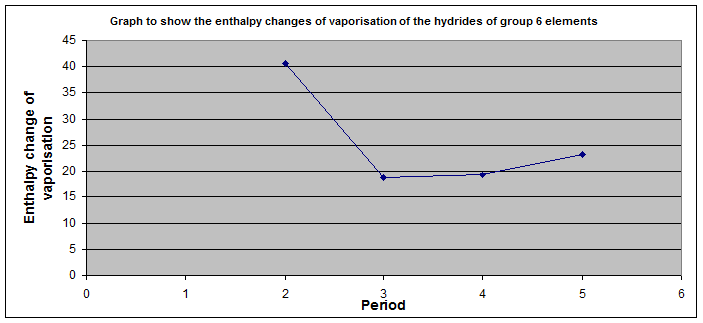
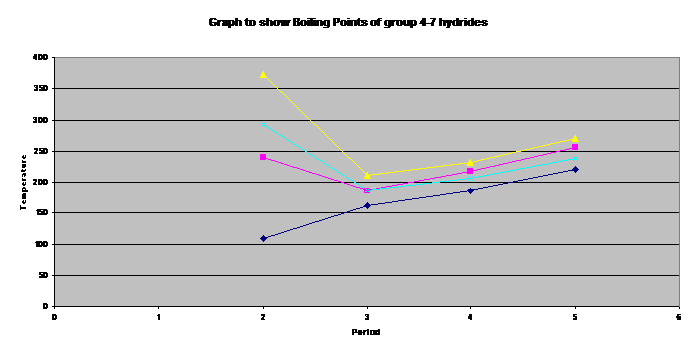
Hydrogen bonding explains these observations.
It is the strongest of the intermolecular forces.
Why are hydrogen bonds so strong?
The atoms O,N,F are so strongly electronegative that the bonding pair of electrons are so far from the H that they are almost able to be donated.
This along with the small size of the H atom means that the H in the molecule is very positive.
The pair of bonding electrons are very near the O,N,F. With their small sizes they are very negative.
With its own lone pair(s) of electrons a strong force of attraction is able to occur between the H and the lone pair of electrons on neighbouring molecules.
This force of attraction is known as a Hydrogen bond and is represented by a dotted line:
 |
|
|
Summary |
1) Within a molecule, the hydrogen must be highly polarised (very positive)
2) Within a molecule, the atom joined to the hydrogen must be very electronegative, O,N,F.
3) Within a molecule, the atom joined to the hydrogen must also have a lone pair of electrons.
H
O,N,F
O,N,F must have a lone pair
Hydrogen bonding is strong enough to change physical properties but not chemical properties.
Water would be a gas at room temperature and pressure if it was unable to hydrogen bond.
Ice is less dense than water:
 |
|
 |
 |
|
|
|
 |
|
Properties of giant metallic lattices:
1) High melting and boiling points -
Attraction occurs between the fixed ions and the delocalised electrons.
The attraction between the positive ions and the 'sea of electrons' is strong
2) Good electrical conductors -
 |
|
3) Malleability and ductile -
Malleable - can be hammered or pressed into shape.
Ductile - can be drawn / stretched into wire.
Due to the delocalised electrons, the metallic structure has a degree of 'give' which allow layers to slide past each other.
Alloys
These are mixtures of metals. This do not however form compounds.
Compounds have a definite ratio of the elements.
Metals can be mixed in different proportions.
In the structure one metal ion is replaced with another.
Often one ion is bigger than the original metal ion.
This acts as a barrier preventing the layers from sliding past each other.
This makes the alloy harder than the original metal.
Q1-3 P 67
Giant ionic lattices
 |
|
Properties of ionic compounds
1) High melting and boiling points:
There are strong electrostatic forces of attraction between the ions.
This means that they are not easily broken which is why they have high melting and boiling points.
The higher the charges between the ions, the stronger the electrostatic forces of attraction.
The stronger the forces of attraction, the more heat energy is required to overcome those forces and hence melt / boil.
2) Electrical conductivity:
In a giant ionic lattice, the ions are held in a fixed position. This means that the ions cannot move.
This is why they do not conduct electricity as a solid.
When the ions are molten or dissolved - the ions are now free to move.
This means they will conduct electricity:
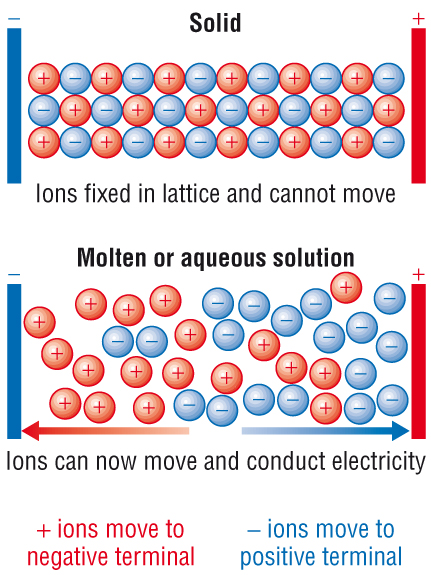
Solubility:
When an ionic solid dissolves, the ions in the lattice separate.
The energy required to separate ions within a lattice is large. ie high melting points.
The energy required on dissolving must be equal and opposite to the energy required to separate ions (as dissolving separates the ions).
Where does the energy come from?
Where does this large amount of energy come from if all we are doing is dissolving the solid in water?
There must be some process during dissolving that can release enough energy to separate the ions from the lattice.
If energy is being released there must be some type of attractive interaction to release energy:-
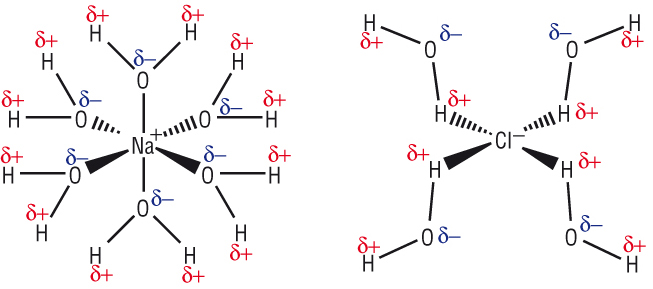
The force of attraction comes between the d+ / d- end of the polar water molecule and the opposite charges on the ion.
This attraction releases energy (as all ‘bond forming’ reaction do).
Many water molecules surround the anion as shown.
This releases energy which is used to break up the lattice structure:
Qu 1-3 P69
Structures of covalent compounds
Covalent compounds fall into 1 of 2 categories:
A) Simple molecular lattice
B) Giant covalent lattice
A) Simple molecular structures:
These are made up from simple (small) molecules such as: CO2, N2, O2, I2 and H2O.
In its solid forms, the molecules are held together by weak intermolecular forces (VDW / Dipole / H Bonding).
The atoms within the molecules are made up from strong covalent bonds.
 |
 |
 |
 |
| Diamond | Graphite |